Marinosolv® enables a strongly increased solubilization and thereby enhanced bioavailability of hydrophobic small molecules and peptides. Consequently, more efficient treatments of a multitude of diseases can be envisaged. Marinosolv® technology facilitates targeted drug delivery with a low systemic off-target activity. Existing drugs and off-patent active ingredients can be improved and re-patented as part of new formulations using Marinosolv®.
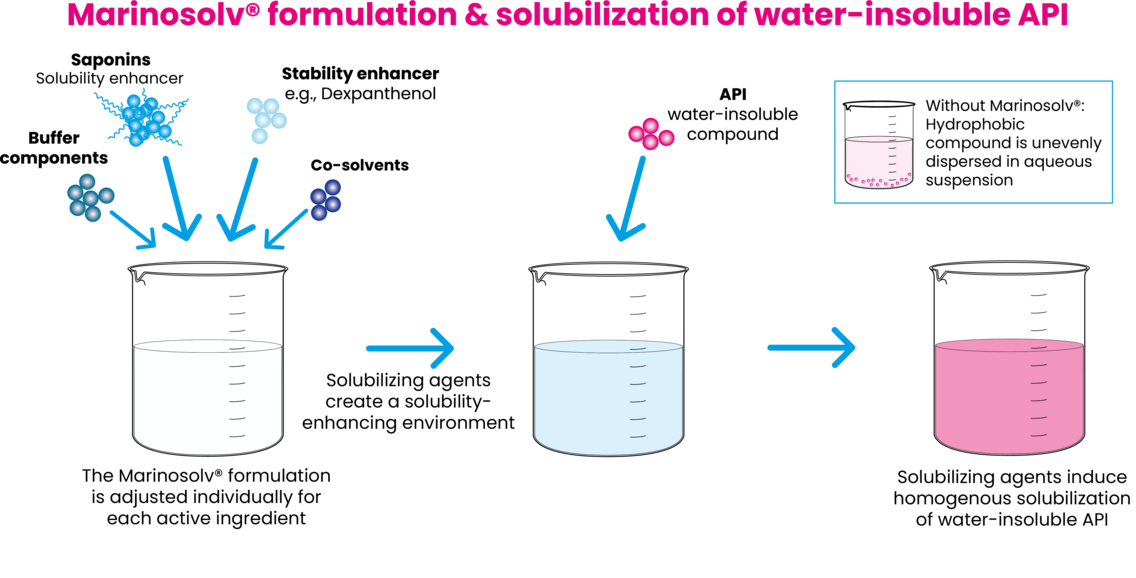
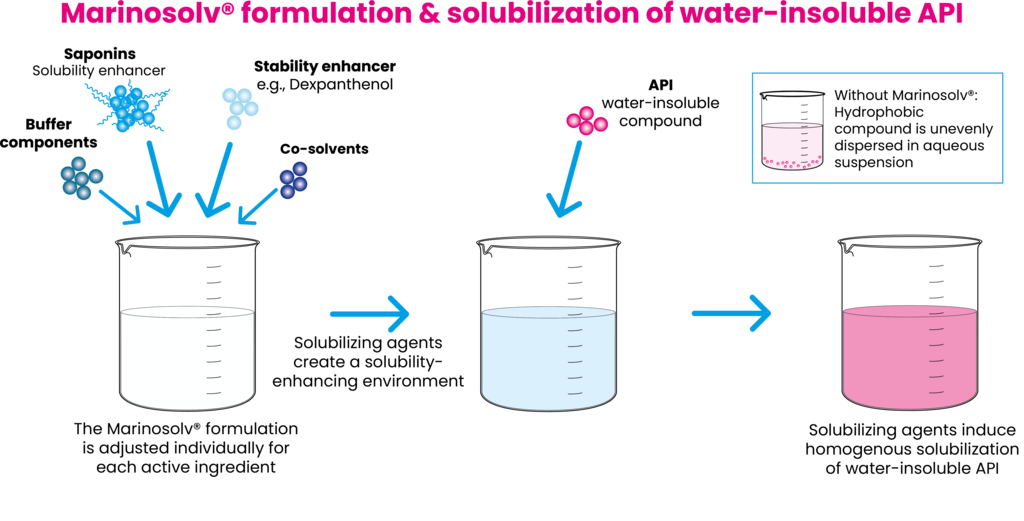
Saponins (e.g. Escin, Glycyrrhizin), Dexpanthenol and selected solvents are used as solubility-enhancing agents in aqueous formulations. The different constituents of the Marinosolv® matrix are individually composed in buffered solutions for an optimized formulation of each drug substance.
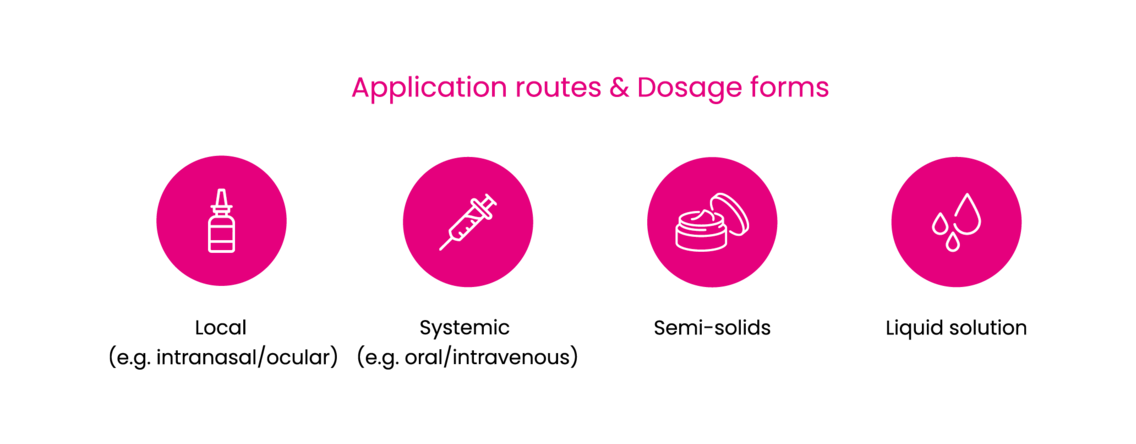
Using Marinosolv® as formulation technology offers several new applications for highly insoluble substances in eyes, lungs, as well as several other sensitive target tissues, resulting in high local activity and low systemic side effects. Marinosolv® may be used for re-formulation and/or re-purposing of established API's enabling life cycle extension of locally applied, locally acting drugs but also for systemic administration routes.
