Formulation development
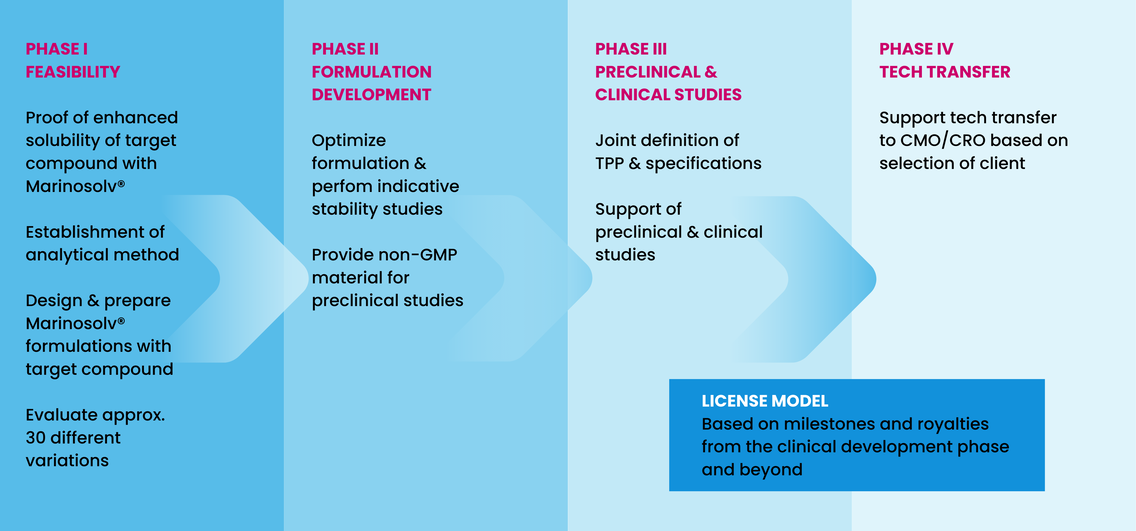
Our formulation development specialists provide you the optimal dosage form for your API and application, based on our Marinosolv® technology. Through this Marinosolv® technology we address solubility and bioavailability issues of hardly soluble small molecules and to support formulation development of Active Pharmaceutical Ingredients in all stages of drug development.